In a major multi-institutional study, Indian scientists found that the HIV-1 virus in India is changing in ways that make powerful antibodies less effective. These viruses, common in India, respond differently compared to those in South Africa. While some antibodies still work well, others don’t. The researchers say that this means we can’t rely on a one-size fits all approach. They call for regular tracking of how the virus is evolving to make sure prevention methods stay effective.
This study has been published in the Journal of Virology, American Society for Microbiology. https://doi.org/10.1128/jvi.00008-25
The main institution involved in this study is The Translational Health Science and Technology Institute (THSTI), Faridabad and from the University of Hyderabad (UoH) Prof. Anand K Kondapi, Dean, School of Life Sciences and Senior Professor, Department of Biotechnology and Bioinformatics is part of this research.

Prof. Anand K Kondapi
Abstract
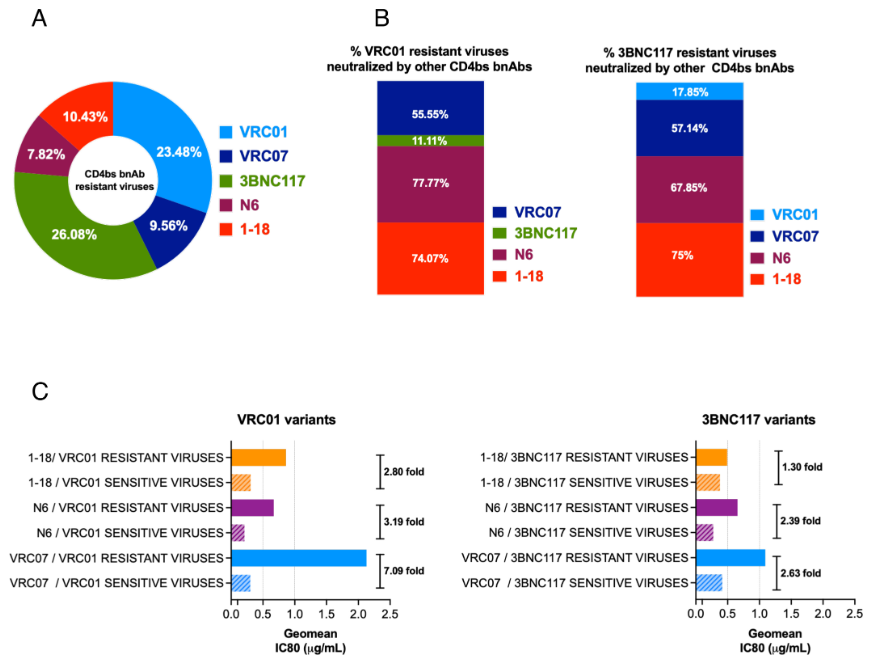
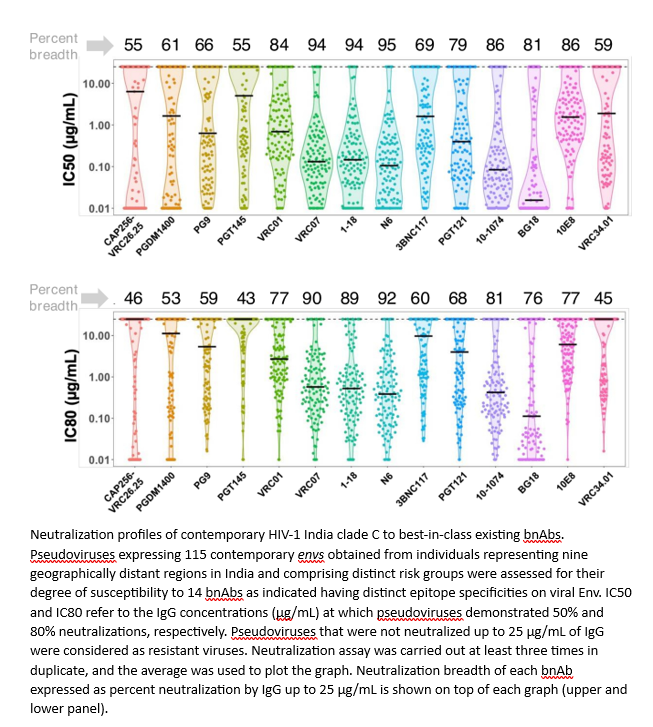
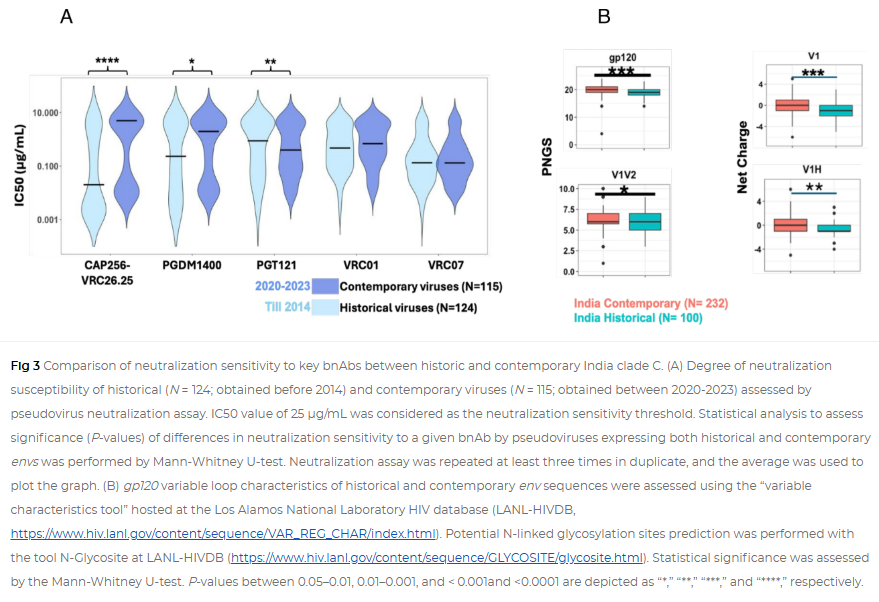
While broadly neutralizing antibodies (bnAbs) have been clinically shown to prevent HIV-1 acquisition, their relative effectiveness against regionally relevant HIV-1 forms is not clear. In the present study, we examined the extent of neutralization susceptibility of contemporary HIV-1 Indian clade C at a population level along with a head-to-head comparison with that from South Africa against a panel of clinically relevant best-in-class bnAbs. Env-pseudotyped viruses encoding HIV-1 India clade C env were found to be best neutralized by the V3 glycan-directed bnAbs (10-1074 and BG18) and select CD4 binding site (CD4bs)-directed bnAbs (VRC07, N6, and 1-18); however, they demonstrated significant resistance to V1/V2 apex-directed bnAbs. Interestingly, the magnitude of the neutralization sensitivity differed between contemporary India and South Africa clade C. Neutralization resistance to key bnAbs was observed to be associated with differences in residues on Env that form bnAb contact sites, gp120 loop lengths, and potential N-linked glycans. Notably, the second generation CD4bs bnAbs (VRC07, N6, 1-18) showed neutralization of VRC01- and 3BNC117-resistant viruses but with two- to sevenfold reduced potency compared to the VRC01-sensitive counterparts, likely due to the enrichment of resistance-associated residues observed in loop D. Predictive analysis indicated that the combination of BG18, N6, and PGDM1400 can provide over 95% neutralization coverage of contemporary India clade C at 1 µg/mL (IC80), an observation distinct from that observed with Africa clade C. Our study clearly highlights that both the complementarity of bnAb classes and the regionally relevant HIV-1 forms are important in achieving clinical effectiveness.
Importance
While the development of vaccines to prevent HIV infection remains a global priority, their potential effectiveness is limited by the extraordinarily diversified circulating forms of HIV-1. The prospect of best-in-class broadly neutralizing antibodies (bnAbs) as a potential prevention option has been demonstrated in several studies, including the phase 2b Antibody-Mediated Prevention trials; however, to be broadly applicable, bnAbs will need to overcome the substantial variability of HIV env circulating globally, beyond the regions where efficacy trials are conducted. The present study highlights that the region-specific contemporary HIV-1 clade C viruses not only vary in their degree of susceptibility to the best-in-class clinically relevant bnAbs, but also are evolving at a population level to become increasingly resistant to the best-in-class bnAbs. Overall, the outcome of this study highlights the need for periodic assessment of sequence and neutralization profiles of the circulating regionally relevant HIV-1 forms toward prioritizing the bnAb combination suitable for effective intervention.
Ethics Approval
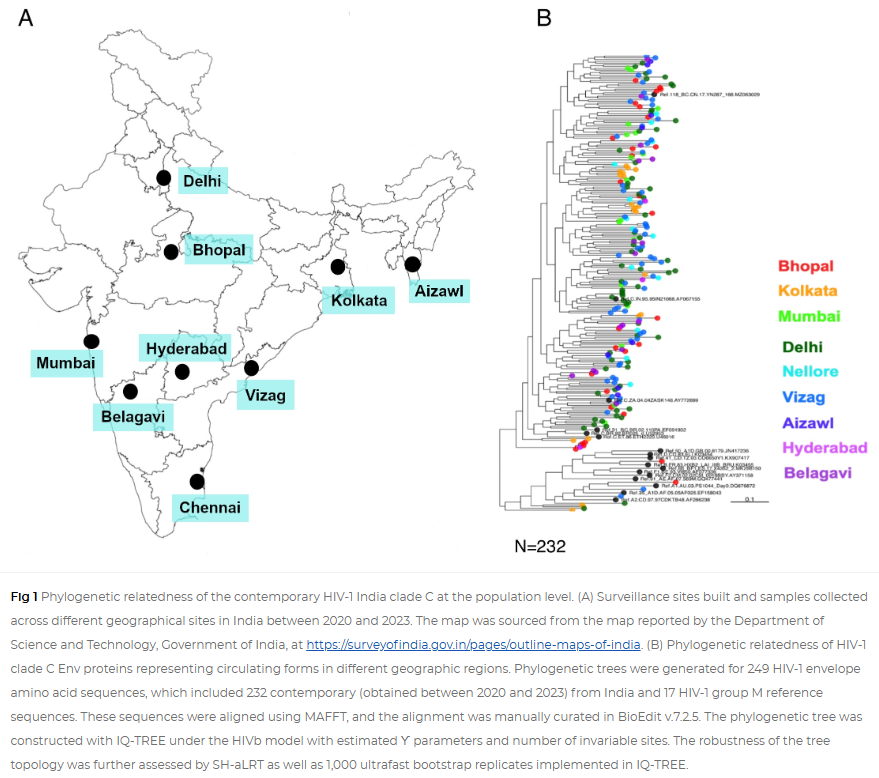
All clinical samples from nine different sites in India were obtained following approval from respective institutional ethical committees. Written informed consent forms in English and local languages were provided and duly signed by all the recruited study participants. Experiments at respective institutions were initiated post approval of the institutional ethics committee. All experiments were carried out at the THSTI, Faridabad post approval of institutional ethics committee (IEC) and institutional biosafety committee. HIV-1-infected individuals were recruited from nine geographically distinct clinical sites in India. They are from Eastern (Kolkata), Western (Mumbai, Belagavi), Northern (Delhi, Bhopal), Southern (Nellore, Hyderabad, Vizag), and North Eastern (Aizawl), following approvals from the institutional ethics committee at all participating institutions.